Introduction to Particle Physics Part 1: What is the world made of?
(This is the first in a 4-part introductory series on Particle Physics, a new part will be released daily after November 26th)
Our view of the composition of the world has changed remarkably over the centuries. By the middle of the 1800s, 60 different substances called elements had been discovered, out of which the entire world was thought to be made. Each element was believed to be completely different from all the rest, though no one knew why at the time. However, in the 1860s a number of brilliant scientists began to spot patterns that allowed the elements to be grouped together into a single structure – the periodic table of the elements. For the first time, we had an ordered structure hinting that there could be a common explanation behind these building blocks.
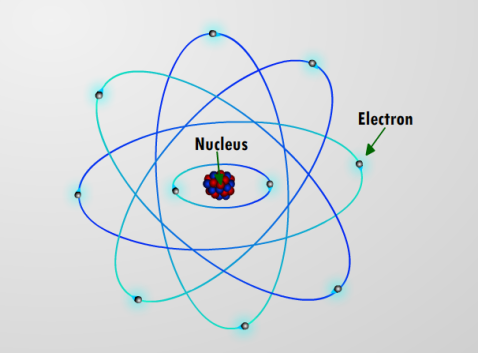
In 1897, a huge breakthrough was made when JJ Thompson discovered a brand new fundamental particle – what we now call the electron. This was followed in 1911 with Ernest Rutherford’s ‘planetary model’ of the atom, with a dense positively charged nucleus (made of particles called protons) surrounded by orbiting negatively charged electrons. There was a missing ingredient though, as some atoms of the same element were observed to be heavier than others. The final piece of the puzzle came in 1932, when J. Chadwick discovered a new particle with no charge – the neutron. And with that discovery, we finally had an explanation for all the elements in the periodic table: each element has a different number of protons, and the reason they behave differently is that they have different numbers of electrons. Elements were no longer the basic building blocks; the number of fundamental particles had been reduced to just 3!
But even before it was complete, the simple planetary model was already being replaced by a better theory of nature: the Quantum Theory. This solved many of the great problems facing Physics in the 1920s, but it faced much opposition due to its counter-intuitive nature. Quantum Mechanics allows particles to be in many places at once, objects to pass through solid walls and things to pop in and out of existence from empty space! It tells us that almost all of the matter making up our world is actually empty space – a deep insight into how reality can surprise us. But the surprises didn’t stop there, oh no, this was just the beginning…
(Next time: what holds the world together?)


How do we zoom in to count branches? I am not clear on that. Thank you.
LikeLike
You can zoom in on talk (and see all three views of the event) after sharing, but not on the main classify page. This is to avoid biasing results depending on whether certain users meticulously zoom in whilst others choose not too.
LikeLike
It’s hard to tell if a vertex is off center in the slice views because you don’t have depth.
LikeLike